Common Formats Background

The Agency for Healthcare Research and Quality (AHRQ) coordinates the development of Common Formats for reporting and analysis of patient safety data. This activity is authorized by the Patient Safety and Quality Improvement Act of 2005 (Patient Safety Act) and the Patient Safety and Quality Improvement Final Rule (Patient Safety Rule). A major goal of the legislation is to allow aggregation of data to identify and address underlying causal factors affecting patient safety and quality. This legislation also called for the establishment of Patient Safety Organizations (PSOs) and a Network of Patient Safety Databases (NPSD) to aggregate and analyze patient safety data.
For more information on PSOs, the Patient Safety Act, and the Patient Safety Rule, please see AHRQ's PSO website: https://pso.ahrq.gov/.
AHRQ has developed Common Formats for three settings of care - hospitals, community pharmacies, and nursing homes. The Common Formats for Diagnostic Safety are the only Common Formats for Event Reporting that are applicable across healthcare settings. In addition, AHRQ is seeking comment on a new version of the Common Formats for Surveillance - Hospital for use in monitoring patient safety outcomes in the hospital setting.
Common Formats for Event Reporting - Diagnostic Safety (CFER-DS)
As part of the agency's efforts to improve diagnostic safety and quality in healthcare, AHRQ has released the Common Formats for Event Reporting - Diagnostic Safety Version 1.0 (CFER-DSV1.0). The CFER-DS is intended to help healthcare providers collect data for analysis of Diagnostic Safety Events in a standardized manner across healthcare settings and specialties for the purpose of learning about how to improve diagnostic safety and better support clinicians in the diagnostic process. Widespread use of the CFER-DS will make it possible to collect, aggregate and analyze diagnostic safety-related information from healthcare providers across the country, which in turn will accelerate learning in this important area of patient safety.
Common Formats for Event Reporting - Hospital (CFER-H)
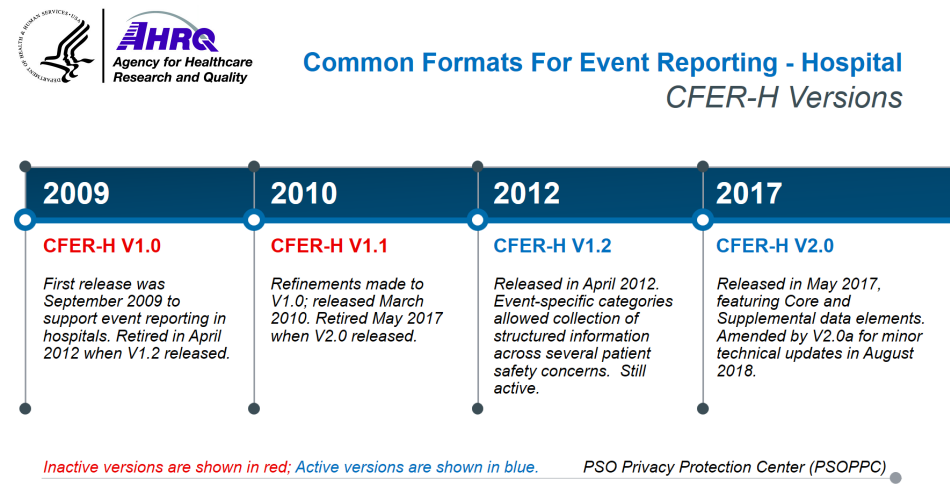
After completing a review of the existing patient safety reporting systems from a variety of health care organizations, AHRQ developed, piloted, and released Common Formats - Hospital Version 0.1 Beta (CF-H V0.1 Beta) in August 2008. Using feedback obtained from both private sector and individuals, AHRQ released CFER-H V1.0 in September 2009.
Further refinements were made to the Hospital Common Formats, incorporating public comments on Version 1.0, which led to the creation of Common Formats for Event Reporting - Hospital Version 1.1 (CFER-H V1.1) in March 2010. In April 2012, AHRQ rolled out Common Formats for Event Reporting - Hospital Version 1.2 (CFER-H V1.2), which included event descriptions, sample reports, and forms for both generic and event-specific categories. The Generic Hospital Common Formats forms included the Healthcare Event Reporting Form (HERF), Patient Information Form (PIF), and Summary of Initial Report (SIR), and specified collected information pertaining to all patient safety concerns. The event-specific categories for CFER-H V1.2 allowed the collection of structured information across several patient safety concerns.
Common Formats for Event Reporting - Hospital Version 2.0 (CFER-H V2.0) constituted a major release of the AHRQ Common Formats. CFER-H V2.0, published in May 2017, incorporated new and modified content as well as technical specifications revised since the release of CFER-H V1.2. CFER-H V2.0 represented an overall decrease in scale from CFER-H V1.2 to reduce the reporting burden. CFER-H V2.0 consolidated the HERF, PIF, and SIR forms into one module, called the Generic module. This version also eliminated paper forms to encourage electronic reporting of patient safety concerns. Additionally, Core vs. Supplemental data elements were defined, with Core being content required for event reporting to the PSOPPC for national aggregation and analysis, and Supplemental being for optionally collected data by providers for additional analysis that could be reported to the PSOs but would not be accepted by the PSOPPC for national aggregation and analysis. In August 2018, CFER-H V2.0a was released with minor technical changes.
Common Formats for Event Reporting - Hospital release timeline:
Common Formats for Surveillance - Hospital (CFS-H)
In February 2014, AHRQ released Common Formats for Surveillance - Hospital Version 0.1 Beta (CFS-H V0.1 Beta), which includes both generic and event-specific categories. The event-specific categories for patient safety surveillance in CFS-H are designed to provide data on adverse events collected through retrospective review of medical records. These formats facilitate improved detection of events and calculation of adverse event rates in populations reviewed. In November 2019, AHRQ released Common Formats for Surveillance - Hospital Version 0.3 Beta (CFS-H V0.3 Beta), which added the Hospital Acquired Condition (HAI)-Other along with the existing HAIs. An HAI-Other event is an infection other than those HAIs identified in the specific HAI Event Descriptions (EDs). The latest version of the Common Formats for Surveillance - Hospital Version 1.0 includes refined data definitions and a new hospital-acquired Coronavirus module.
Common Formats for Event Reporting - Nursing Home (CFER-NH)
AHRQ released the Common Formats - Nursing Home Version 0.1 Beta (CF-NH V0.1 Beta) in March 2011, including event descriptions, sample reports, and forms for generic and event-specific categories. Based on the feedback received through public comments and consultation with various stakeholders in August 2019, AHRQ released Common Formats for Event Reporting - Nursing Home Version 1.0 (CFER-NH V1.0), which allows Patient Safety Organizations (PSOs) or vendors to submit patient safety concerns to the PSOPPC and to the Network of Patient Safety Databases (NPSD).
Common Formats for Event Reporting - Community Pharmacy (CFER-CP)
AHRQ released the Common Formats - Retail Pharmacy Version 0.1 Beta in October 2015. AHRQ worked with the NQF based on the public review and comment received, and developed and released the Common Formats for Event Reporting - Community Pharmacy Version 1.0 (CFER-CP V1.0) in December 2016. The CFER-CP V1.0 module is designed for use in the community pharmacy environment to gain enhanced understanding about the circumstances surrounding patient safety data in the community pharmacy setting. This module is self-contained, covering everything necessary to report patient safety data in the Community Pharmacy event-specific category.
Patient Safety Concerns - What Gets Reported?
Patient Safety Concerns include any circumstance involving patient safety, and encompasses patient safety events (both incidents and near misses) and unsafe conditions.
- Incident: A patient safety event that reached the patient, whether or not the patient was harmed.
- Near miss (or close call): A patient safety event that did not reach the patient.
- Unsafe condition: Any circumstance that increases the probability of a patient safety event.
AHRQ Common Formats include:
- A common set of definitions of patient safety concerns that may give rise to patient harm
- Examples of patient safety sample reports
- Paper forms for certain Common Formats versions such as the Common Formats for Event Reporting - Diagnostic Safety Version 1.0 (CFER-DS V1.0), to guide development of data collection instruments
- A Users Guide which describes how to use the Formats
- A meta-data registry with data element attributes and technical specifications for use by developers
- Is evidence-based
- Harmonizes across governmental health agencies
- Incorporates feedback from the private sector
- Structured: Structured data permits sorting of patient safety incidents and near misses for event analysis, as well as for pattern and trending analysis at all levels of the healthcare system. Structured data encompasses important descriptors, known risk factors, and the use of established risk reduction methods to permit efficient analysis of incidents and patterns of patient safety events. Structured data can be aggregated within and across provider organizations, as well as for national reports. Such data are de-identified, where appropriate, in accordance with the Statute and Regulations.
- Narrative: Narrative information cannot be aggregated, but provides the necessary details about an individual event or condition needed to understand patient safety concerns at the provider and/or PSO levels. The narrative information may also assist with how the provider and/or PSO can act to reduce risk to patients.
Active for Reporting
Only active versions of the Common Formats are accepted by the Patient Safety Organization Privacy Protection Center (PSOPPC).
CFER-H V1.2, released in April 2012, incorporates all of the content updates made to the Common Formats and includes full technical specifications for electronic implementation.
CFER-H V2.0, released in May 2017, incorporates all new and modified content applied to the Common Formats for Hospitals and provides full technical specifications and supporting documentation for implementation. A minor technical release (CFER-H V2.0a) was implemented in August 2018. CFER-H V2.0 is also available for submission through the Single Report Data Submission (SRDS) tool.
Both CFER-H V1.2 and CFER-H V2.0 are active for reporting to the PSOPPC and for implementation and use by healthcare providers in hospitals and PSOs.
CFER-CP V1.0, released in December 2016, is the first version of Common Formats for the Community Pharmacy setting and is active for reporting, implementation, and use by community pharmacies and PSOs.
CFER-NH V1.0, released in August 2019, is the first version of Common Formats for Nursing Home setting and is active for reporting, implementation, and use by nursing homes and PSOs.
CFER-DS V1.0, released in May 2022, is the first version of Common Formats for Diagnostic Safety. CFER-DS V1.0 can be used across all healthcare settings. It is active for use by healthcare providers and PSOs and is also available for submission through the SRDS tool.


